Membrane technologies for hydrogen separation
DOI 10.12910/EAI2021-021
by Silvano Tosti – Head of Nuclear Technologies Laboratory- ENEA, Alessia Santucci - Nuclear Technologies Laboratory – ENEA; Alfonso Pozio - Energy Storage, Batteries and Technologies for Hydrogen Production and Utilization Laboratory - ENEA
Separation technologies based on membranes can be applied in continuous processes and are characterized by system design flexibility (easy scaling-up and hybridization with conventional processes), capability to produce high-quality products and saving energy. Membranes consisting of thin-walled Pd-Ag tubes have been developed by ENEA for recovering hydrogen isotopes in the fusion fuel cycle (hydrogen and tritium separation from He, water detritiation) and for producing ultra pure hydrogen via reforming and other dehydrogenation reactions in green chemistry applications.
Le tecnologie di separazione a membrana possono essere applicate con successo a processi continui per ottenere prodotti di alta qualità e risparmiare energia, grazie alla loro flessibilità, alla facilità di accrescerne le dimensioni e di abbinarli a processi convenzionali. L’ENEA ha sviluppato tubi in lega di palladio a parete sottile che possono essere utilizzati per recuperare e/o separare gli isotopi dell’idrogeno nell’ambito del ciclo del combustibile dei reattori a fusione. In particolare, questi dispositivi consentono la separazione di idrogeno e trizio dall’elio e la detriziazione dell’acqua e trovano anche applicazione nella produzione di idrogeno ultrapuro attraverso il reforming e altri processi di de-idrogenazione nel campo della chimica verde.
Separation technologies based on membranes can be applied in continuous processes and are characterized by system design flexibility (easy scaling-up and hybridization with conventional processes), capability to produce high-quality products and saving energy. In this vein, the features of the membrane technologies result to be fully sound with the requirements of the green chemistry processes. Membranes consisting of thin-walled Pd-Ag tubes have been developed by the ENEA Frascati laboratories for recovering hydrogen isotopes in the fusion fuel cycle (hydrogen and tritium separation from He, water detritiation).
Pd-membrane reactors resulting from the combination of membrane tubes with a catalyst bed can exhibit high reaction conversions thanks to the continuous removal of the hydrogen, one of the reaction products. Based on their capability to separate hydrogen from gas mixtures with an infinite selectivity, these Pd-based tubes, developed in the framework of the fusion fuel cycle activities, have then been applied for producing at lab-scale ultra-pure hydrogen (e.g. suitable for feeding polymeric fuel cell) through dehydrogenation reactions.
The Department for Fusion and Technologies for the Nuclear Safety conducts at Frascati laboratories the R&D activities on fusion energy, mainly supported by the European program (European Fusion Development Agreement, 1999-2013, and Consortium EUROfusion, 2014-present).
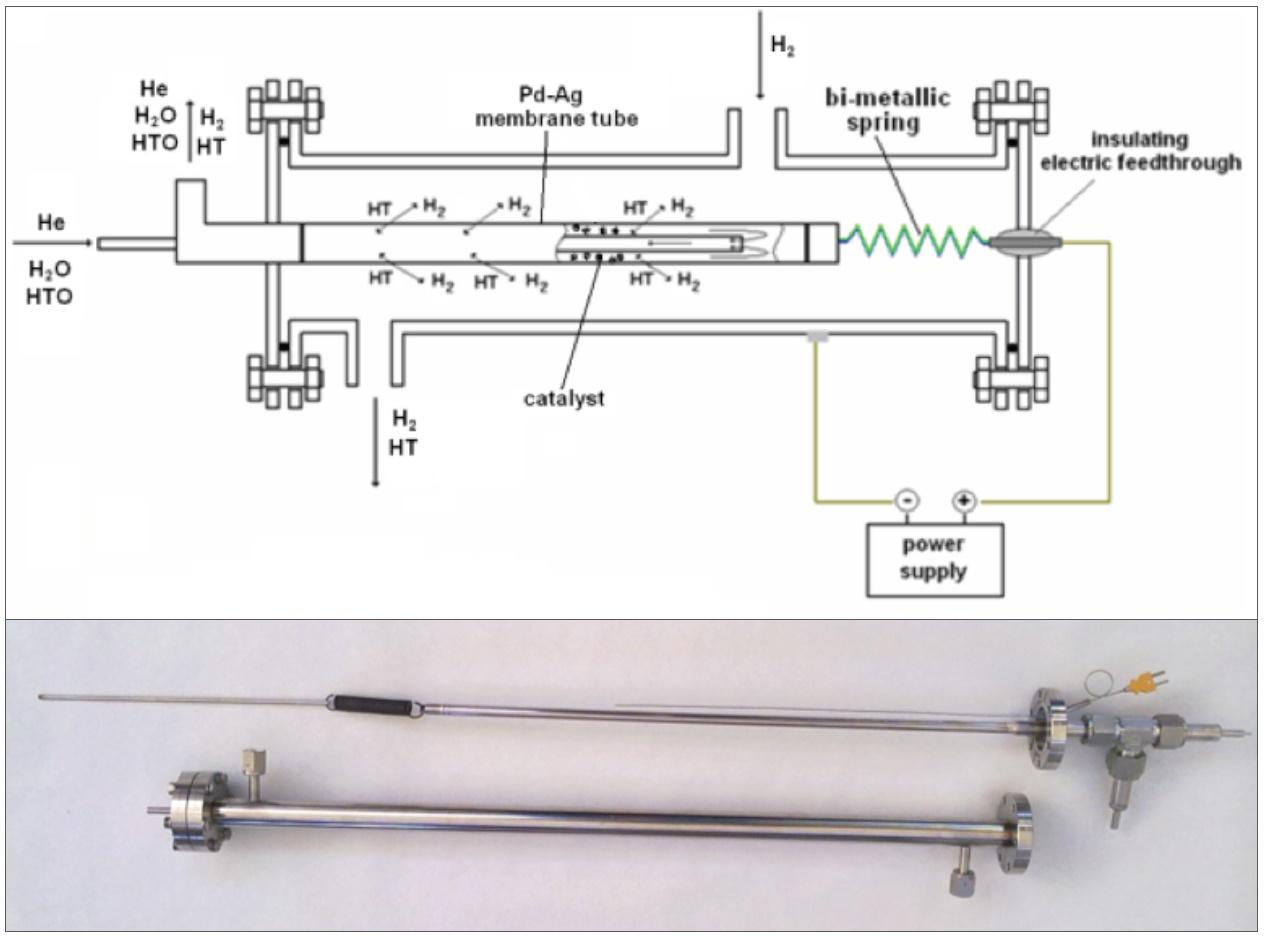
One of the main activity carried out at ENEA Frascati laboratories has concerned the development of separation units dedicated to the extraction and purification of the hydrogen isotopes. Such processes are a part of the fusion fuel cycle aimed at providing the deuterium and tritium needed to sustain the fusion reaction [1, 2]. The research activities were focused on the design, construction and characterization of thin-walled Pd-tubes that exhibit the capability of separating with infinite selectivity hydrogen and its isotopes from gas mixtures [3, 4]. The Pd-tubes were used both as permeators and as membrane reactors for recovering tritium via gas permeation, water gas shift and isotopic exchange reactions [5, 6]. As an example, Fig. 1 shows a Pd-membrane reactor studied for the recovering of tritium from tritiated water via isotopic exchange.
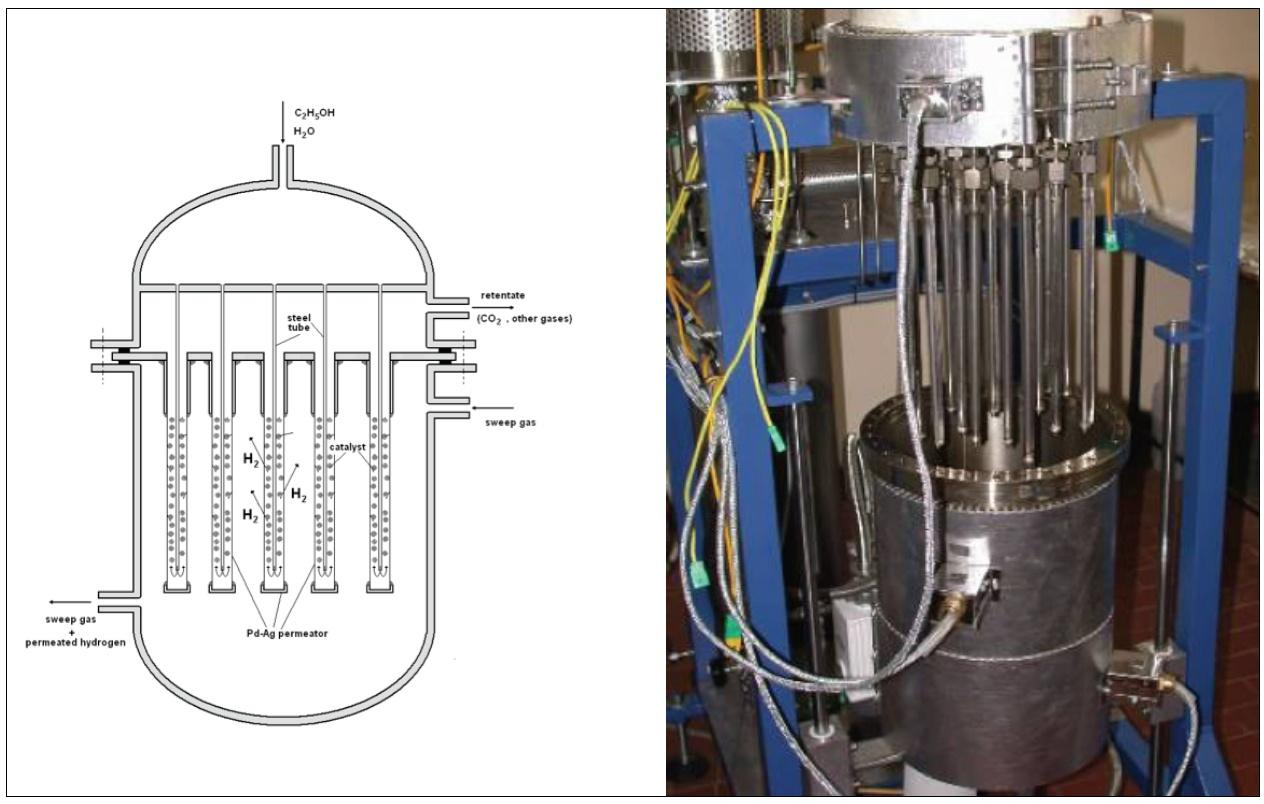
An innovative Pd-membrane reactor and a process have been developed with the CEA (Commissariat a l’energie atomique et aux énergies alternatives) for the recovery of tritium from tritiated waste of the JET (Joint European Torus in operation at Culham, UK) [7-9]. Following this collaboration, Pd-membrane reactors designed for detritiation processes have been produced by ENEA laboratories and delivered to CEA in the frame of commercial agreements: Fig. 2 reports the internal view of a membrane reactor consisting of 8 Pd-tubes. In a recent study, an innovative application of porous membranes (acting as a membrane gas-liquid contactor) was proposed for the extraction of tritium from liquid breeder of fusion reactors [10, 11].
Ultra-pure hydrogen
Further studies carried out in strict collaboration between Frascati and Casaccia research centers demonstrated that the Pd-membranes developed for the fusion fuel cycle exhibit the capability to produce ultra-pure hydrogen via dehydrogenation of hydrocarbons, alcohols and biomass. In principle, a Pd-membrane reactor, a combination of a Pd-membrane with a catalyst, is capable to perform dehydrogenation reactions with very high conversions [4]. In fact, the continuous removal of one of the products (i.e. the hydrogen) promotes the reaction conversion according to the well-known “shift effect” of the membrane. Main applications have concerned the production of hydrogen via reforming of methane, ethanol and biomass. Fig.3 shows the scheme and the picture of a membrane reactor made of thin-walled Pd-tubes for producing pure hydrogen via ethanol reforming. In particular, from the reforming of olive mill wastewater (OMW), a by-product of the olive oil industry with significant pollution effect in the Countries of the Mediterranean regions, it is possible to produce hydrogen and syngas [11-13]. OMW reacts over a catalyst bed inside Pd-tubes while the hydrogen produced is extracted through the membrane: some kg of hydrogen can be produced per ton of OMW while the content of organic compounds in the retentate of the membrane reactor is reduced by more than 90%.
The reforming of methane with OMW has been studied through a reforming unit consisting of multi-tube membrane reactors and capable to produce up to 1 m3/h of hydrogen [13]. In the frame of the project Microgen30 (call Industria2015), the high-grade hydrogen produced by these membrane units has been thought to feed polymeric fuel cells of cogeneration systems where electricity and heat were produced from methane with high efficiency.
Hydrogasification of biomass
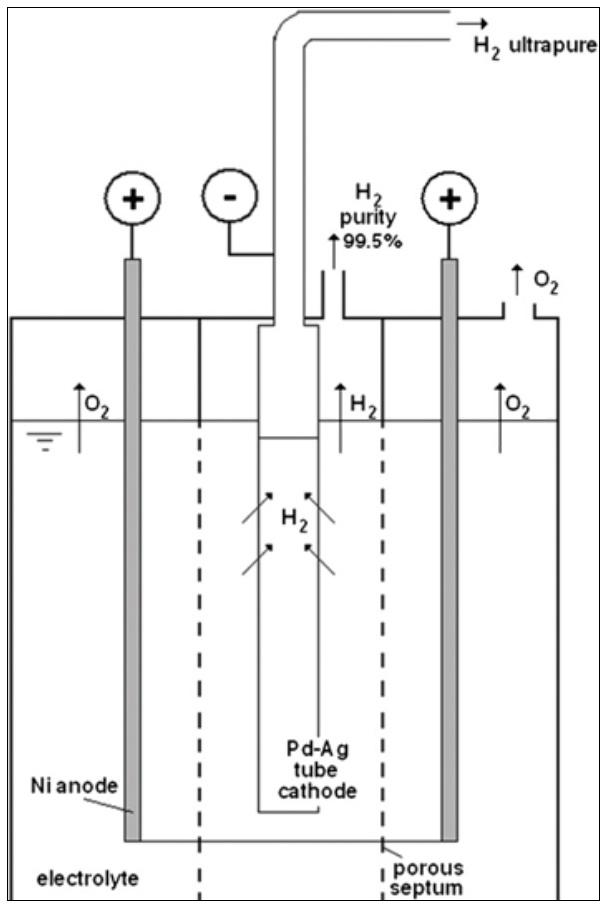
Another application shows the use of Pd-based tubes, that exhibit complete selectivity to hydrogen, in small scale applications for purifying the hydrogen produced by the alkaline electrolysers. A study dedicated to these applications has developed an alkaline electrolyser where the cathode consisted of a Pd-membrane tube as shown in Fig. 4 [14, 15]. In this device, about 50% of the hydrogen produced by electrolysis is directly extracted through the membrane tube as ultra-pure hydrogen without any need of further purification.
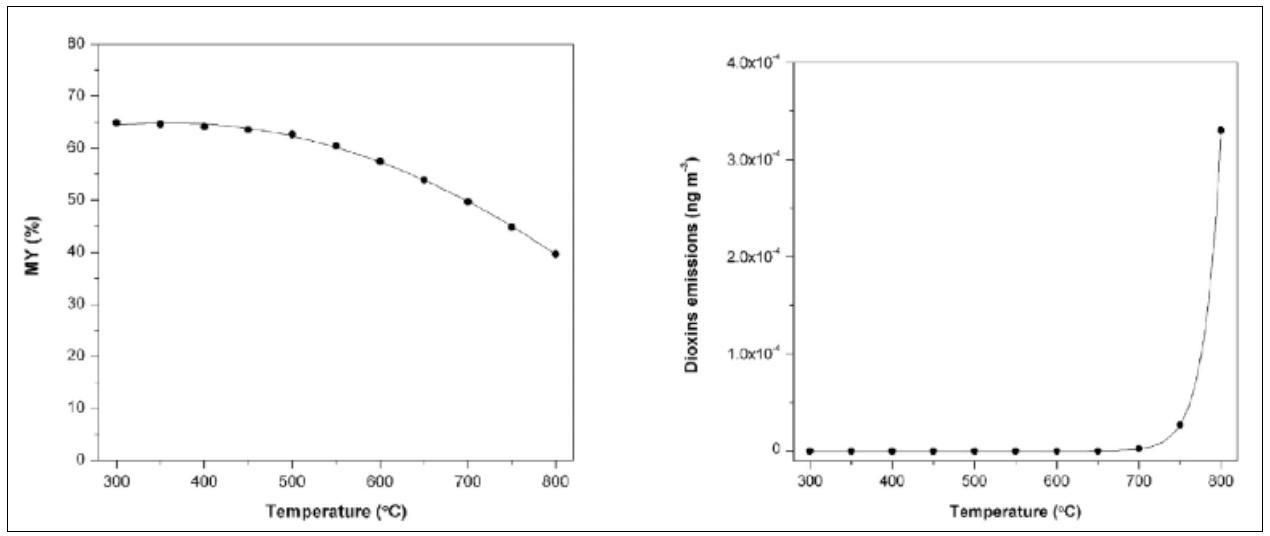
Other studies considered the hydrogasification of biomass to produce methane enriched streams [16]. The hydrogasification of refuse derived fuel (RDF) is a waste-to-fuel process characterized by negligible toxic emissions (e.g. dioxins and furans) typical of the other thermal processes used for the treatment of this biomass (e.g. incineration, gasification, etc.) [17, 18]. The operation with excess of hydrogen allows to achieve high methane yields and practically zero emissions of dioxins as reported in Fig. 5 related to the case of a hydrogasification reactor fed with 1000 kg h-1 of dry RDF and a hydrogen excess of about 40 kg h-1.
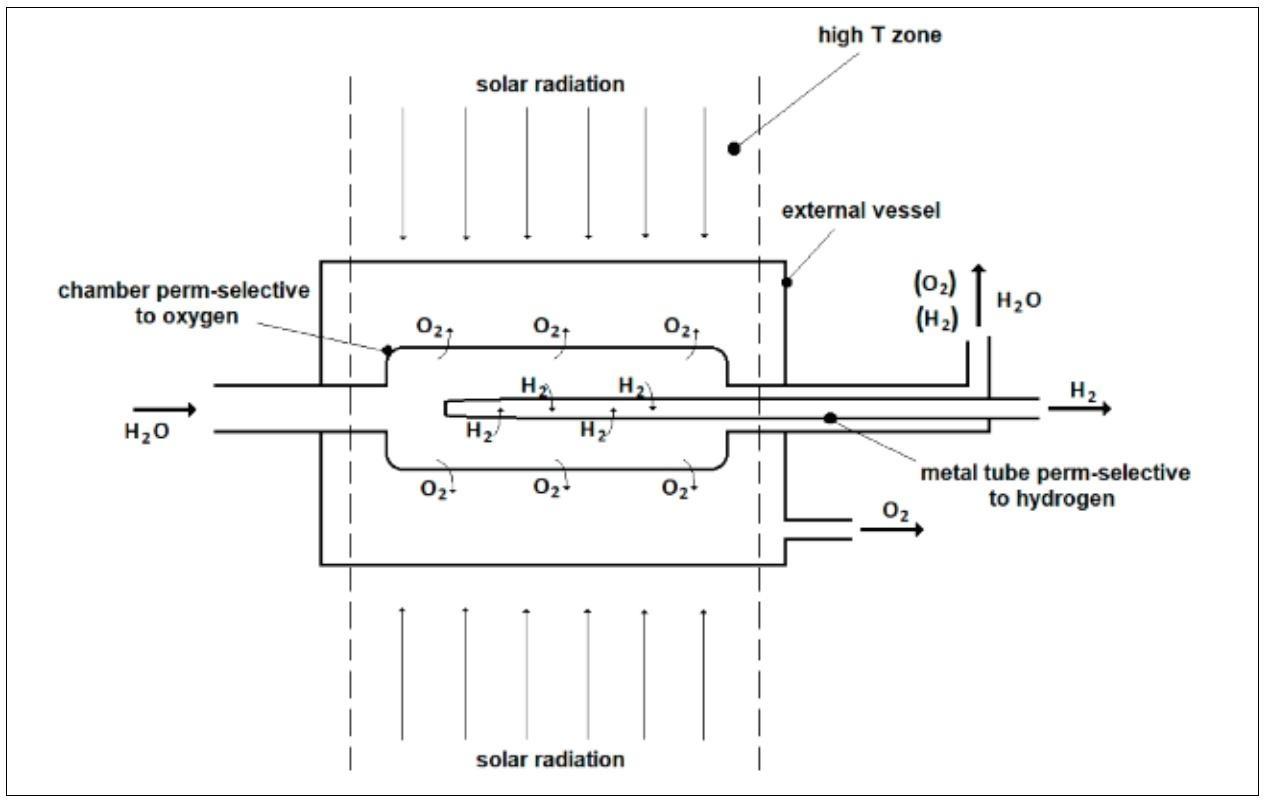
A recent analysis was addressed to the use of an innovative reactor for the direct thermal splitting of water. This membrane reactor consists of two distinct membranes for the extraction of the hydrogen and the oxygen respectively as schematically reported in Fig.6 [19, 20]. This study has shown that the presence of a couple of membranes amplifies the “shift effect” typical of a membrane reactor thus allowing the reduction of the process temperature. In a case study considering a membrane reactor made of Ta membrane tubes of wall thickness 0.15 mm and a chamber perm-selective to oxygen made of hafnia of thickness 0.1 mm, reaction conversions of 20% are obtained by this membrane unit at around 2000 °C, much below the temperature of 2700 °C needed to a traditional reactor for attaining the same performances. At 2000 °C, about 200 and 100 Nm3/h of pure hydrogen and pure oxygen are respectively produced by feeding 1000 kg h-1 of water and assuming a membrane permeation efficiency of 0.9. The reaction conversion of about 10% is achieved by this membrane reactor at 1800 °C, a temperature that could make feasible the exploitation of solar energy for the direct production of hydrogen and oxygen from water.
Projects and perspectives
Presently, the high cost of the precious metal used for manufacturing the thin-walled Pd-tubes is consistent with their applications in the fusion fuel cycle. Outside fusion technologies, these Pd-membrane units have found only niche applications where high hydrogen purity is required. Typical examples are laboratory alkaline electrolysers where the hydrogen purification can be efficiently performed by Pd-membranes. The perspective of using these metal membranes on large scale devices is based on the development of less expensive metal alloys instead of the Pd-based ones [21]. In this vein, the studies on binary and ternary alloys based on refractory metals are expected to demonstrate the viable and economic applicability of dense metal membranes for producing pure hydrogen via reforming of biomass. In fact, as verified with the Pd-membranes, this kind of membrane reactors could operate in continuous processes by exhibiting high reaction conversions as required by the future processes of the “hydrogen chain”.
Main technological transfer actions are expected within the sectors of the treatment of the OMW and the RDF hydrogasification. ENEA is discussing collaborations with Italian enterprises of these sectors with the aim to complete the industrialization of the processes studied through the realization and optimization of prototypical installations.
The R&D activities on the Pd-membranes in the fusion fuel cycle are mostly carried out in the frame of the EUROfusion program that is funding the European research on fusion energy. The Frascati laboratories plays a prominent role for the realization of membrane processes to extract hydrogen isotopes from He (used both as coolant and purge gas in breeding blankets) and from tritiated water. Main collaborations are with the partners of the EUROfusion Consortium, in particular the laboratories of the Karlsruhe Institute of Technology (DE) and the CEA-Cadarache Center (FR). The use of Pd-membranes for recovering tritium from gaseous streams are currently proposed also inside the project TRANSAT aimed to select cross-cutting technologies for tritium processing in fusion and fission reactors.
Conclusions
The collaboration between the departments FSN and TERIN has allowed the development of a sound know-how around Pd-based membranes and membrane processes for the separation of hydrogen and its isotopes. Several characteristics of the membrane processes (continuous operation, modularity, reduction of sizes, capability to work with high reaction conversion and then high energy efficiency) are the pivotal requirements for the fusion fuel cycle applications since they have to operate safely and with low tritium inventory. The challenge is to develop metal alloys cheaper than those Pd-based, with the aim to candidate the separation technologies based on metal membranes for supporting the penetration of the hydrogen as an efficient and safe energy vector in the future sustainable energy scenarios. Processes for producing hydrogen from renewable sources (e.g. reforming of biomass, solar-assisted water splitting) or exploiting the renewable hydrogen to produce green fuels (e.g. hydrogasification of RDF) could be applications where to demonstrate the results of R&D activities on metal membranes
REFERENCES
- S. Tosti and N. Ghirelli editors, Tritium in Fusion: Production, Uses and Environmental Impact, Nova Science Publishers, Hauppauge, NY, USA: 2013, ISBN 978-1-62417-270-0
- S. Tosti and A. Pozio, Membrane Processes for the Nuclear Fusion Fuel Cycle, Membranes 2018, 8, 96; doi:10.3390/membranes8040096
- S. Tosti, L. Bettinali, D. Lecci, F. Marini, V. Violante, Method of bonding thin foils made of metal alloys selectively permeable to hydrogen, particularly providing membrane devices, and apparatus for carrying out the same, European Patent EP 1184125 (European patent Application n. 01830465.9 del 12.7.2001, Grant on 11.07.2012)
- S. Tosti, Overview of Pd-based membranes for producing pure hydrogen and state of art at ENEA laboratories, International Journal of Hydrogen Energy 35 (2010) 12650-12659
- S. Tosti, C. Rizzello, F. Borgognoni, N. Ghirelli, A. Santucci, P. Trabuc, Design of Pd-based membrane reactor for gas detritiation, Fusion Engineering and Design 86 (2011) 2180-2183
- A. Santucci, R. Antunes, G. Bruni, F. Mallozzi, M. Incelli, M. Sansovini, Recent achievements of the Pd-Ag membrane technologies in tritium extraction system applications, Fusion Engineering and Design 146 (2019) 2242-2246
- S. Tosti, N. Ghirelli, F. Borgognoni, P. Trabuc, A. Santucci, K. Liger, F. Marini, Membrane reactor for the treatment of gases containing tritium” Patent Application PCT/IT2011/000205 del 16.06.2011 – European Patent Grant EP 2582618 del 14.05.2014
- N. Ghirelli, S. Tosti, P. Trabuc, F. Borgognoni, K. Liger, A. Santucci, X. Lefebvre, Process for the detritiation of soft housekeeping waste and plant thereof, Patent Application Domanda di Brevetto Internazionale PCT/IT2011/000211 del 21.06.2011, European Patent Grant EP 2586034 del 25.03.2015
- X. Lefebvre, P. Trabuc, K. Liger, C. Perrais, S. Tosti, F. Borgognoni, A. Santucci, Preliminary results from a detritiation facility dedicated to soft housekeeping waste, Fusion Eng. Des. 87 (2012) 1040-1044
- A. Pozio, S. Tosti, F. Marini, Processo a supporto poroso per l’estrazione di idrogeno ed isotopi da metalli liquidi, e relativo apparato”, Italian Patent Grant n. 102018000003185 del 23.03.2020
- S. Tosti, A. Pozio, L. Farina, M. Incelli, A. Santucci, D. Alique, Membrane gas-liquid contactor for tritium extraction from Pb-Li alloys, Fusion Engineering and Design 158 (2020) 111737
- S. Tosti, M. Sansovini, A process for treating waste waters of oil mills by means of reforming reaction, and plant thereof – PCT Application WO 2014/073014 A1 – European Patent Grant 13829002.8 15.02.2017
- S. Tosti, C. Cavezza, M. Fabbricino, L. Pontoni, V. Palma, C. Ruocco, Production of hydrogen in a Pd-membrane reactor via catalytic reforming of olive mill wastewater, Chemical Engineering Journal 275 (2015) 366–373, http://dx.doi.org/10.1016/j.cej.2015.04.001
- S. Tosti, M. Fabbricino, L. Pontoni, V. Palma, C. Ruocco, Catalytic reforming of olive mill wastewater and methane in a Pd-membrane reactor, International Journal of Hydrogen Energy 41 (2016) 5465-5474
- A. Pozio, S. Tosti, L. Bettinali, R. Borelli, M. De Francesco, D. Lecci, F. Marini, Elettrolizzatore alcalino con catodo tubolare in Pd-Ag per la produzione di idrogeno ultrapuro, Attestato di brevetto n. 0000271920 del 19.10.2011
- A. Pozio, M. De Francesco, Z. Jovanovic, S. Tosti, Pd-Ag hydrogen diffusion cathode for alkaline water electrolysers, International Journal of Hydrogen Energy 36 (2011) 5211-5217
- Fabio Borgognoni, Silvano Tosti, Monia Vadrucci, Alessia Santucci, Combined methane and ethanol reforming for pure hydrogen production through Pd-based membranes, International Journal of Hydrogen Energy 38 (2013) 1430-1438
- S. Tosti, G. Buceti, A. Pozio, Process and related plant for the production of methane from refuse-derived-fuel, Italian Patent Grant n. 102017000086210 del 05.12.2019, European Patent Application n. 18185183.3 del 04.05.2020
- S. Tosti, M.A. Sousa, G. Buceti, L.M. Madeira, A. Pozio, Process analysis of refuse derived fuel hydrogasification for producing SNG, International Journal of Hydrogen Energy 44 (2019) 21470-21480
- S. Tosti, A. Pozio, L. Farina, A. Santucci, Processo a membrana per la produzione di idrogeno ed ossigeno mediante idrolisi dell’acqua e relativo apparato”, Italian Patent Application n. 102020000023470 depositata il 06.10.2020
- S. Tosti, A. Pozio, L. Farina and A. Santucci, Hydrogen and Oxygen Production via Water Splitting in a Solar-Powered Membrane Reactor—A Conceptual Study, Hydrogen 2021, 2, 18–32. https://doi.org/10.3390/hydrogen2010002
- A. Santucci, S. Tosti, A. Basile, “Alternatives to palladium in membranes for hydrogen separation: nickel, niobium and vanadium alloys, ceramic supports for metal alloys and porous glass membranes”, in Handbook of membrane reactors, volume 1, ed. A. Basile, Woodhead Publishing Series in Energy – Cornwall (UK), 2013, Ch. 4, pp. 183-217.