Separation chemistry: tool to improve the recovery of raw materials from waste
In this paper the application of separation processes to improve recycling materials has been analyzed from the economical and environmental viewpoint
Chimica delle separazioni: uno strumento per migliorare il recupero di materie prime dai rifiuti
Per ridurre l’impatto delle attività economiche sull'ambiente sono necessari cambiamenti radicali e mirati. Il supporto di tecnologie pulite è pertanto necessario per stimolare la transizione verso un'economia sostenibile. Da questo punto di vista le tecnologie di separazione, o meglio ancora la chimica delle separazioni può dare un vantaggio efficace e, in particolare, dove "riciclo" e/o "minimizzazione" hanno la priorità. Nell’ambito del trattamento dei rifiuti l’utilizzo di tecniche separative efficienti permette di recupere materie prime preziose salvaguardando le risorse naturali. Il miglioramento dell’efficienza dei processi produttivi rappresenta inoltre un’opportunità ulteriore per essere competitivi in un mercato caratterizzato da eccesso di offerta
In questo lavoro sono riportati esempi di applicazione di processi di separazione utilizzabili nell’ambito del trattamento e riciclaggio di rifiuti
Loris Pietrelli
Introduction
The degree of environmental stress is widely known and it is clear that the present level of impact of the human activities on the environment cannot be provided without causing unacceptable damage to our planet. From the end of the last century many Institutions have made the same warning: “we cannot continue our present methods of using energy, managing forests, farming, protecting plants and animal species, managing urban growth and producing industrial goods” [1]. The spontaneous reaction of the scientific community to balance industrial development and sustainable use of natural resources, was the creation of the “industrial ecology” concept [2]. It seems to be a contradiction in terms probably because we use to consider the industrial systems as separate from the environment but production and consumption activities interacts with the surrounding ecosystems and environment in general, by introducing many additional chemical and physical phenomena. Methods such as Life Cycle Assessment (LCA), carbon and water foot-printing are increasingly popular. In particular to investigate the opportunities to change material and product flows in an environmentally compatible direction.
On the other hand, one reason of the difficult in implementing the sustainability and the “industrial ecology” is that the approach in the “Economy” still tend to the opening of the material cycles, wasting resources. Some powerful drivers start to be considered as catalyst for the industry interest in developing practical tools for environmental conservation. Legislation, employee involvement, health and safety programs, market pressure, etc. seems to become the modern driving forces for the industrial development.
The environmental legislation and market pressure, at moment, should be considered as the most important driving forces for the sustainable development because they strongly force companies to recycling materials and to reduce waste, and toxic materials. Market for environmentally improved products and services are growing but unfortunately, not as fast as the environment requires. At moment introduction of technology innovation seems to be the most important tool to obtain sustainable development. This means that the road to innovation and environmental protection requires a great deal of individual ecletism as well as managerial efforts, needed to obtain both optimization of the production processes and pollution reduction.
The purpose of this paper is to discuss some of the important pursuit of separation processes in terms of controlling and reducing the impact of the industrial production. It is not the intention of this paper to be an all-inclusive or critical review of activities on the field of waste management, but rather to point out some directions and to suggest technical options that are necessary to make sustainable development a practical reality.
Separation chemistry
The recent advances in the understanding transport phenomena, new development in equipped design and greater demand of high quality products (therefore environmentally compatible) have spurred growth and diversification in the field of separation processes.
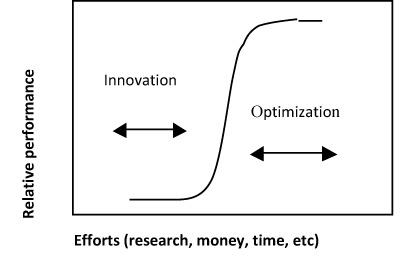
To understand why we have to consider “separation technologies” a tools for the sustainable development and in particular for waste reducing, we may take a look at Figure 1 which shows a typical Relative Performance vs. Effort curve. Such type of curves characterized the development of many separation techniques (as well as of many other technologies). For scientists and technicians it is easy to appreciate that at moment we could operate at the breakthrough part of the curve and we have the great opportunity to achieve a lot with a relative minor efforts.
New adsorbent materials, extractant and membrane systems for use in a variety of old and new applications, and new solvents for use in extraction or adsorption are among the advances that have led to changes in traditional methods for selecting, designing and operating separation processes.
Not only heavy metals removal necessary before discharging wastewater to the environment, but recovery of non-renewable resources (water itself) represent a significant benefit both in terms of economic and environmental protection. To fit high quality wastewater treatment to improve recycling, separation process represent an important tool. For example, conventional metal removal from wastewater is carried out by a variety of techniques such as precipitation, membrane separation, ion exchange, adsorption, solvent extraction etc (Table 1). There are excellent reviews available describing these “mature” technologies and the benefits and disadvantages associated with each one.
Industrial wastewater, for example, show extremely variable chemical and physical features produced by mixing solution and/or suspension. According to the end-of-pipe approach it makes more difficult the selective separation of material (compounds) eventually reusable. While, following a flow chart and introducing the best available separation technique within the productive process, an effective reduction of pollutants can be done.
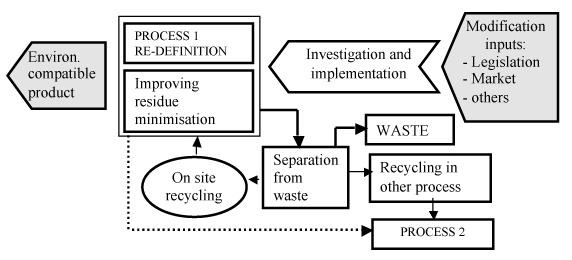
The sustainable development could be performed mainly through a Pollution Prevention Strategy that usually consists of some steps such as: redesign process, minimize wastes, reuse of process residues, design process so that the residue streams become material for the same or other processes (“cascade of use”) according the scheme reported in Figure 2. All of these action can be done by introducing new technologies as general approach, and in particular separation step in somewhere of the process or improving the separation efficiency (e.g. substituting obsolete precipitation/flocculation with membrane process). From this point of view a process “flow chart” represent an important inventory tool for the identification of all possible sources of waste generation and the most useful action to reduce waste and/or re-use materials. At list also a sort of waste quality control could be an important tool for process evaluation. The process “flow chart” starts with the identification of the simplest unit operation where materials and energy are input and, after transformation, output. Therefore, by this way, the best available technical option can be chosen.
At moment unfortunately processes developed as sustainable systems cannot operate with economical benefits but it is an effective investment in terms of environmental protection.
TABLE 1
Separation techniques for waste treatment and/or raw material recovery
_________________________________________________________________________________
|
Technique ______________________________________ |
Pollutants ___________________________________ |
|
Solvent extraction |
Phenols, N or Cl Aromatics, Organic acids, Hydrocarbon, Metals, Borate, Cyanides…. |
|
Reverse Osmosis/Ultrafiltration |
Sugars, proteins, Oils, Monomers, Macromolecules, Metals and precipitates, Cell fragments, Bacteria, Suspended mater, etc |
|
Ion Exchange |
Metals, Chromates, mercury, Nitrates, Phosphates, Dyes, Proteins… |
|
Carbons adsorption |
Phenols, N or Cl Aromatics, Aromatics, Cresols, Dyes, Cyanides, Metals. |
Adsorption onto poor materials arsenic, vanadium, fluorine, etc
__________________________________________________________________________________
Technology: case studied
Some examples of processes developed in order to recover raw materials from waste and wastewater are reported.
Rare earth recovery from exhausted batteries [3]
Using a hydrometallurgical process developed for the recovery of metals from spent batteries, a selective separation of RE by precipitation of Sodium RE double sulphate can be performed.
The mixed rare earths were precipitated from the leachate solution, obtained from the batteries treatment, by pH adjustment performed by NaOH addition until pH<1.5 to avoid the ferric hydroxide precipitation usually started at pH 2.5-3. Rare earths are precipitated in the form of insoluble double sulfate salts as confirmed by the X-ray diffraction analysis. The chemistry of the precipitated double sulfates is still somewhat obscure and their composition may be summarized as NaRE(SO4)2xH2O. Solubility of the double sulfates in water are generally low particularly for the cases of La, Ce, Pr, Nd, Pm, Sm, Eu and Sc. As temperature rises solubility falls and moreover the double salt is almost insoluble in saturated solution of the alkali sulphate.
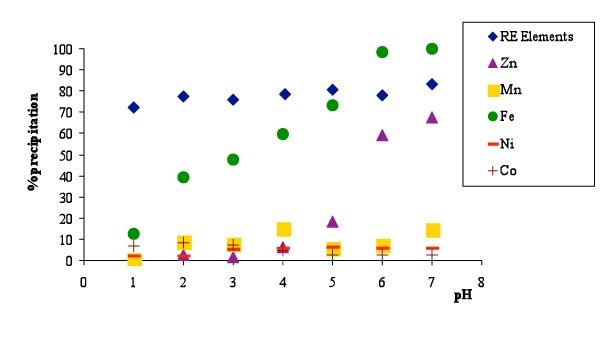
Some of the metals contained in leachate solution can also be precipitated effectively by pH adjustment to give low soluble compounds (ferric hydroxide or manganic oxide) or by co-precipitation or adsorption on a carrier such as iron that usually forms a bulky flock. To obtain RE precipitate with low content of impurities (mainly Fe, Co and Mn) neutralization must be performed slowly and under continuous stirring. With respect to impurities other than RE, the resulting precipitate contains Ni=1.68%, Co= 3.05%, Mn=0.29%, Zn=0.71% and Fe= 6.9%. The loss of Rare Earths after treatment of real waste solution was within 12-18%.
Dyes adsorption onto chitosan [4]
Dyes are widely used in industries such as textile, leather, plastics and paper to colour the final products. Dye producers and users are interested in stability therefore are producing dyestuffs that are more difficult to degrade after use. Since many synthetic dye compounds are harmful to human beings, it is important to encourage industries to implement the colour removal from waste before to discharge. Among several chemical and physical methods, adsorption process on poor materials is one of the most effective to remove dyes from wastewater and chitosan appears to be economically attractive due to its origin: is obtained, on an industrial scale, by the alkaline deacetylation of chitin, the second abundant polymer in nature next to cellulose.
The main commercial sources are waste materials of the seafood industry, mainly shell of crab and shrimp, that are treated with aqueous sodium hydroxide solutions (40-50%) at about 110 °C.
Considering the ion sorption onto chitosan, it is accepted that amine sites are the main reactive groups, though hydroxil groups (especially in the C-3 position) may contribute to sorption. The uptake of dyes by chitosan from solutions involves several steps to transfer the solute from the liquid phase to the specific sites inside the particles (e.g. external diffusion and intraparticle diffusion). The controlling mechanism of the dyes adsorption is mainly depending from the large number and distribution of the –NH2 and –OH groups on the chitosan chains, in particular the correlation coefficient for the pseudo second order adsorption model has extremely high values and its calculated equilibrium adsorption capacity qe,cal fits very well with the experimental qt vales. These suggest that the second order mechanism is predominant and that the overall rate of dye adsorption process appears to be controlled by the chemical process.
TABLE 2
Comparison of kinetic models. T.R.=Telon red, Y.R.= Yellow remazol
|
1st order kinetic model |
2nd order kinetic model |
Intraparticle diffusion model |
|||||||||
|
Ci (mg/l) |
qt (mg/l) |
k1 |
qecal |
R2 |
k2 |
h |
qecal |
R2 |
ki |
R2 |
|
|
T.R. |
341.3 |
170.6 |
2.9x10-2 |
301.8 |
0.858 |
4.1x10-4 |
12.69 |
175.2 |
0.999 |
1.68 |
0.683 |
|
Y.R. |
300.0 |
88.8 |
1.1x10-2 |
36.7 |
0.241 |
4.5x10-4 |
3.86 |
92.6 |
0.994 |
1.53 |
0.370 |
Fluoride removal from wastewater[5]
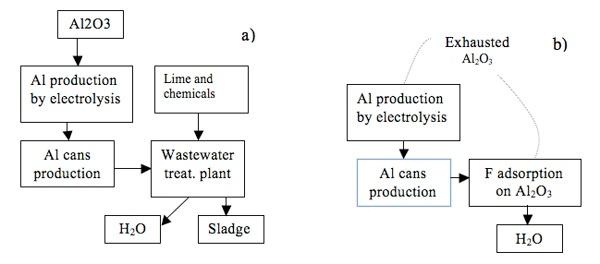
The toxicity of fluoride is well understood and many articles have been published on fluorides removal by using different methods. Activated alumina has been used for many years in municipal water treatment plant such as to sorb HF emission from Aluminum smelting cells or for drinking water.
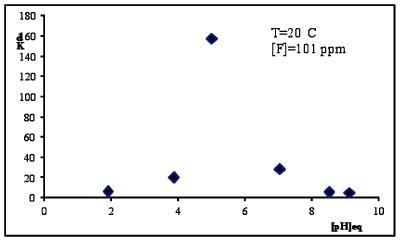
Industries which discharge significant quantities of fluorides in waste streams include aluminum manufacture, particularly washing wastewater. These effluent contains mainly fluoride and aluminum ions, therefore considering the toxicological effect, the Italian legislation requires in the treated effluent the limit of 2 ppm and 6 ppm respectively prior to discharge into the environment. The aluminum is produced by electrolysis of alumina dissolved in a molten cryolite (Na3AlF6) based bath, therefore fluoride removal can be performed by adsorption on the same metallurgical-grade alumina that is subsequently fed to the electrolytic cells of the primary Aluminum-processing industry. The fluoride adsorption by alumina strongly depends on pH (Figure 4) and in particular the highest adsorption capacity occurs at pH=5.5.
The new process gives some advantages such as: simplicity, less capital costs, no sludge has been produced, the economic does not depend on the regeneration of exhausted alumina. In Figure 5 a comparison of the processes is shown.
Copper recovery from WEEE [6]
The development of copper recycling processes from wastes is an important issue to effectively utilize the copper resources. Among the wastes that contain copper, one of the most complex recycling materials is electronic scrap like printed circuit boards (PCB), which generally contain 10–30% copper as well as plastics, fiberglass and other metals. One of the potential processes to recover copper from electronic scrap is copper smelting. However, copper smelters are generally produce air pollution and require high energy consumption. On the other hand, a hydrometallurgical process has lower capital cost and can be economically operated even on a small scale. These advantages enable the construction of an on-site plant for economical recycling without transportation costs.
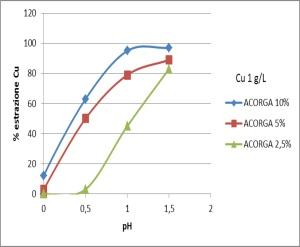
This process uses an nitric salt solution containing copper nitrate and consists of three stages of leaching, purification and electrowinning. Copper in the waste is leached as copper and the impurities in the solution are removed by solvent extraction with Acorga M5640 (the active substance is 2-hydroxy-5-nonylsalicylaldoxime) (Figure 6) and the purified copper solution is electro won (Figure 7).
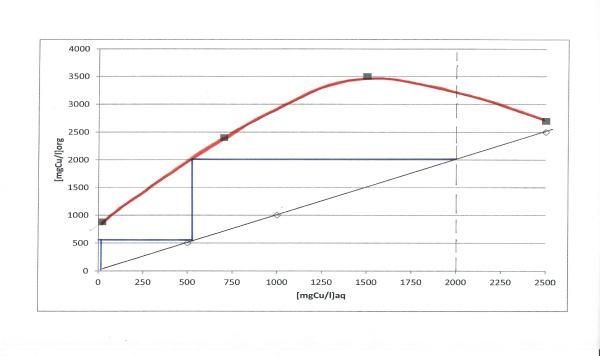
Considering the equilibrium curve diagram to determine the number of theoretical stages (McCabe-Thiele Method) required to achieve a desired degree of separation, the developed extraction method require at least 3 stage (Figure 8) to obtain copper separation from the leaching solution.
Poly(ethylene glycol) removal from wastewater [7]
Poly(ethylene glycol) (PEG) is a highly water-soluble, waxy solid that is used extensively in the cosmetic, pharmaceutical, food, textile, polishing and toiletry industries. Therefore PEGs are commonly found in industrial and domestic wastewaters, as non-ionic surfactants coupled with hydrophobic molecules or as catabolic products of these.
The adsorption behaviour of polymers (and surfactants) on solids strongly depends, generally, on the polymer solution characteristics (i.e. as PEG molecular weight increases, so the polarity weakens and therefore its affinity to water molecules decreases). According to the adsorption studies and considering its large surface area and non-polar surface, there is no ideal universal activated carbon for every application. Hence, it is important to match the properties of the activated carbon with the performance requirements of the process. In particular PEG adsorption is strongly depends from pH and MW.
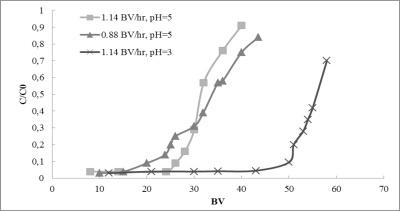
In Table 4 the simplest isotherm model parameters are reported while in Figure 9 the breakthrough curves obtained by using the real wastewater, at different pH and flow rate, are reported.
|
TABLE 4 |
|||||||
|
Langmuir |
Freundlich |
||||||
|
Curve |
Q0 [mg/g] |
KL |
R2 |
RL |
Kf [mg/g] |
1/n |
R2 |
|
PEG-1450, pH=3 |
388.41 |
8.58 |
0.9964 |
0.028 |
353.32 |
0.3540 |
0.8996 |
|
PEG-1450, pH=5 |
188.68 |
5.88 |
0.9836 |
0.055 |
144.97 |
0.3396 |
0.7328 |
|
PEG-1450, pH=8 |
250.71 |
6.67 |
0.9941 |
0.025 |
267.43 |
0.3231 |
0.8843 |
Conclusion
There is a great deal of effort being devoted to the identification of pollution prevention/cleaner technology opportunities. From these separation processes will play an important role as technologies useful for the wastewater treatment and therefore for the sustainable development because they offer a wide selectivity in separation (from suspended particles ad colloids to dissolve ions). Moreover it is of great interest to built up a “Technology Information System” which can carry out a comparative assessment of separation process technologies from a cleaner production point of view.
But how we can begin to introduce these concepts? Probably we have to start at University level because education of engineers and natural scientists became crucial, in order to deal with a serious cultural problem: ecologist usually do not know about the industrial system. However engineers and people from industries in general have a very poor view of nature and are very ignorant about scientific ecology.
In other words we have to consider new separation process schemes to treat wastewater and to recover row materials because the uncontrolled development and irrational exploiting of natural resources (e.g. from many ores the ratio of useful product to waste seldom exceeds 1:10), all too long regarded as freely available and inexhaustible has caused serious ecological disasters.
References
[1] S. Schmidheiny (1992) (with the Business Council for Sustainable Development) Changing Course: Global Business Perspective on Development and the Environment. MIT press, Cambridge, Mass..
[2] R.A. Frosch, N.E. Gallopoulos (1989). Strategies for manufacturing. Scientific American 261 144-152.
[3] Pietrelli L. Fontana D., Bellomo B., Montereali M. (2002). Rare earths recovery from NIMH spent batteries Hydrometallurgy 66:135-139.
[4] L. Pietrelli, T. Carlucci, D. Fontana (2005) Adsorbimento di coloranti industriali su chitosano. V Conf. Int. Valorizzazione e Riciclaggio dei Rifiuti Industriali VARIREI.
[5] Loris Pietrelli (2005) Fluoride adsorption onto metallurgical grade alumina Annali di chimica 95:303-312
[6] Pietrelli L., R. Flammini, M. Pietrantonio, A.Piozzi e I. Francolini (2012). Recupero del rame da schede elettroniche. Atti Ecomondo.
[7] Loris Pietrelli (2013). Effect of MW and pH on poly(ethylene glycol) adsorption onto carbon. Adsorption (in press).
Loris Pietrelli - ENEA, Technical Unit for Environmental Technologies